Introduction:
Vascular anomalies encompass a wide spectrum of disorders defined by abnormal development/growth of blood and/or lymphatic vessels. Medical treatment for vascular malformations has focused mostly on pathways that are involved in abnormal vascular proliferation. Sirolimus, a mTOR inhibitor that is regulated by the PI3K/AKT signaling pathway has been studied as an off-label treatment modality for vascular anomalies, with most studies being centered on the effect of sirolimus in pediatric populations. Few studies have evaluated the safety and efficacy of sirolimus use in treating vascular malformations in adult populations. We developed a single center study to look at adults diagnosed with vascular malformations and the effect of sirolimus.
Methods:
In this retrospective study, we included all adult patients diagnosed with a vascular malformation and prescribed sirolimus for their malformation from January 2000 to July 2023 at Oregon Health & Science University (OHSU). We received IRB approval (OHSU IRB Number: STUDY00025570) to collect patient demographics, pertinent medical history, laboratory values, response to sirolimus, side effects from sirolimus, use of other medications while taking sirolimus, and reasons for discontinuation of sirolimus through individual chart review. Patients were identified by using OHSU's Oregon Clinical and Translational Research Institute Cohort Discovery Tool using the search terms: sirolimus, age >18, and vascular malformations. Additional patients were identified by assessing referrals to the OHSU Hematology Clinic to capture patients not identified with the cohort discovery tool.
Results:
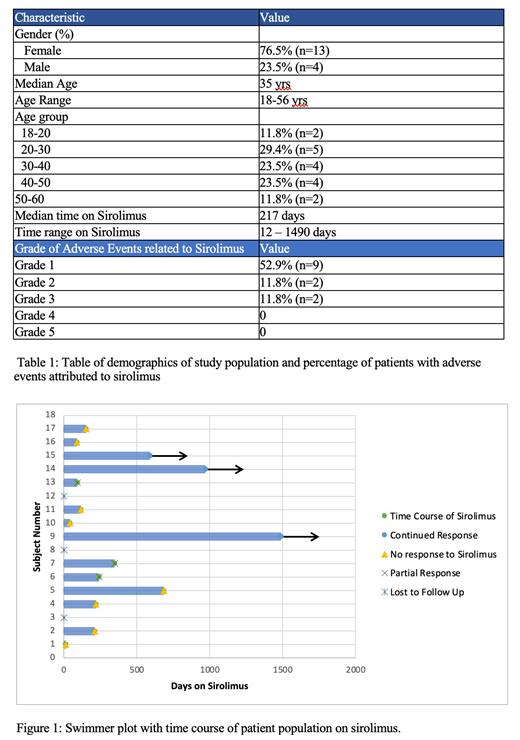
Seventeen patients were identified that met study criteria. The median age was 35 years, with ages ranging from 18-56 years. 23.5% of the patients (n=4) were male and 76.5% (n=13) were female. Of the 17 patients, 10 stopped sirolimus within one year. One patient continued sirolimus for 1.8 years with four patients continuing sirolimus indefinitely. The median time taking sirolimus was 217 days. All patients were started on a dose of 0.8mg/m2 twice daily, with a goal trough level of 8-15mg. Thirteen (76.4%) patients experienced side effects. The most common side effects were mucositis (41.1%, n=7) and GI side effects (17.6%, n=3) such as nausea and/or vomiting. All patients who experienced an adverse event, experienced a CTCAE Grade 3 or less adverse event attributed to sirolimus. Eleven patients (64.7%) experienced a Grade 1 or 2 adverse event and two patients (11.8%) experienced a Grade 3 adverse event. The Grade 3 adverse events included one patient who developed a pulmonary embolism and one patient who developed significant allergy-related angioedema while on sirolimus, which lead to prompt discontinuation. Eleven patients (64.7%) did not perceive any benefit from sirolimus, which eventually lead to discontinuation of sirolimus. Fifteen patients (88%) were followed symptomatically and two patients (11.8%) were followed on imaging. One patient who received MRI imaging after a 9 month course of sirolimus showed interval improvement of their vascular malformation.
Conclusions:
Sirolimus has been suggested to be beneficial to patients who have vascular malformations, although the majority of data has been accrued in pediatric cohorts. Our findings demonstrate that sirolimus is a relatively well tolerated medication in adults, with minimal severe medication related adverse events with only 11.8% of patients experiencing a CTCAE Grade 3 adverse event. However, most adults in our study (64.7%) did not perceive a benefit from sirolimus for their vascular malformations and 58.8% of patients stopped sirolimus within one year. It is still unclear the efficacy of sirolimus for the treatment of vascular malformations in an adult population and more research is needed to fully elucidate the benefits and risks of sirolimus for patients with vascular malformations.
Disclosures
Shatzel:Aronora Inc.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal